Understanding the Periodic Trends in Electron Affinity: A Comprehensive Guide
Understanding the Periodic Trends in Electron Affinity: A Comprehensive Guide
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Understanding the Periodic Trends in Electron Affinity: A Comprehensive Guide. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Understanding the Periodic Trends in Electron Affinity: A Comprehensive Guide
- 2 Introduction
- 3 Understanding the Periodic Trends in Electron Affinity: A Comprehensive Guide
- 3.1 What is Electron Affinity?
- 3.2 Periodic Trends in Electron Affinity
- 3.3 Related Searches:
- 3.4 FAQs about Periodic Trends in Electron Affinity:
- 3.5 Tips for Understanding Periodic Trends in Electron Affinity:
- 3.6 Conclusion
- 4 Closure
Understanding the Periodic Trends in Electron Affinity: A Comprehensive Guide
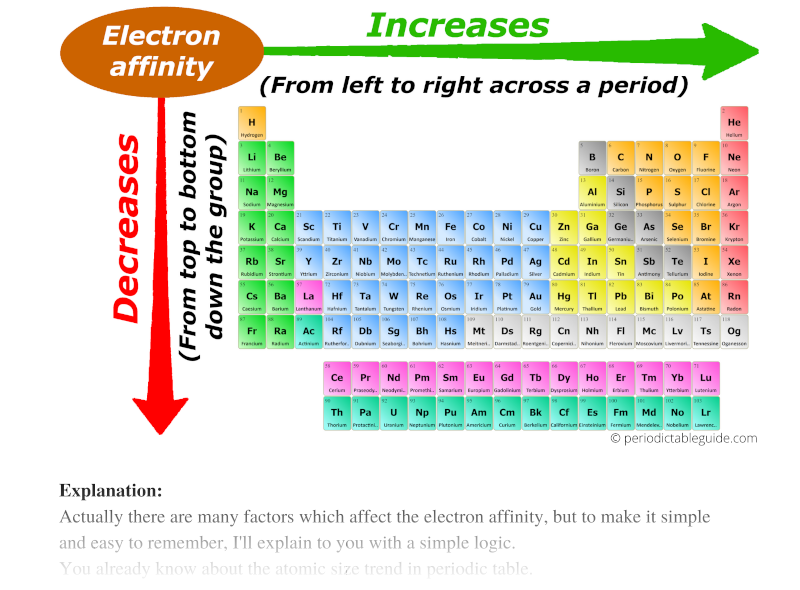
The periodic table, a cornerstone of chemistry, organizes elements based on their properties and reveals predictable patterns in their behavior. One such crucial property is electron affinity, which quantifies an atom’s tendency to gain an electron. Understanding the periodic trends in electron affinity is essential for comprehending chemical reactivity and predicting the formation of chemical bonds.
What is Electron Affinity?
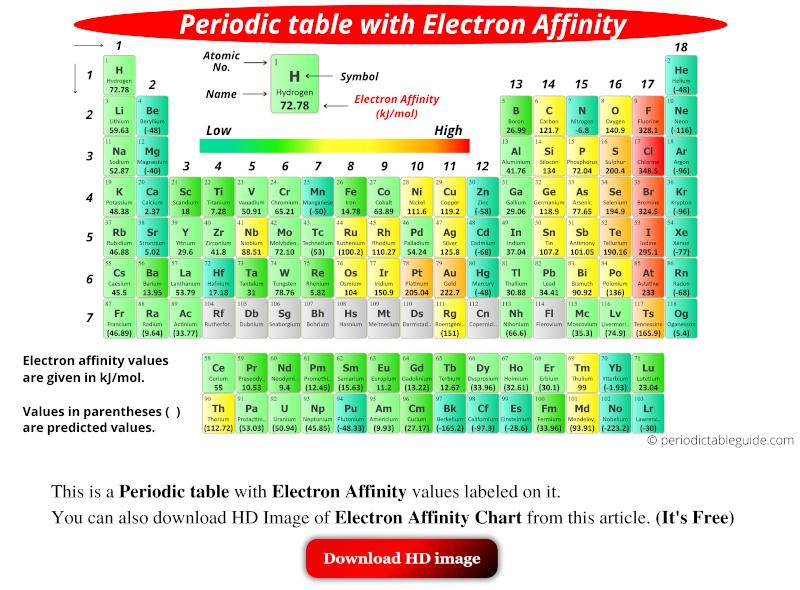
Electron affinity (EA) refers to the change in energy when an electron is added to a neutral gaseous atom to form a negative ion. A higher EA value indicates a greater tendency for the atom to accept an electron, resulting in a more stable negative ion.
Periodic Trends in Electron Affinity
The periodic table showcases a systematic variation in electron affinity across periods and groups. Here’s a breakdown of the key trends:
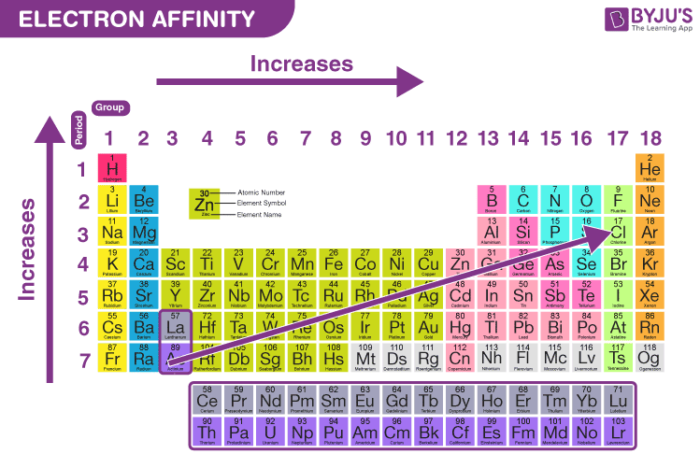
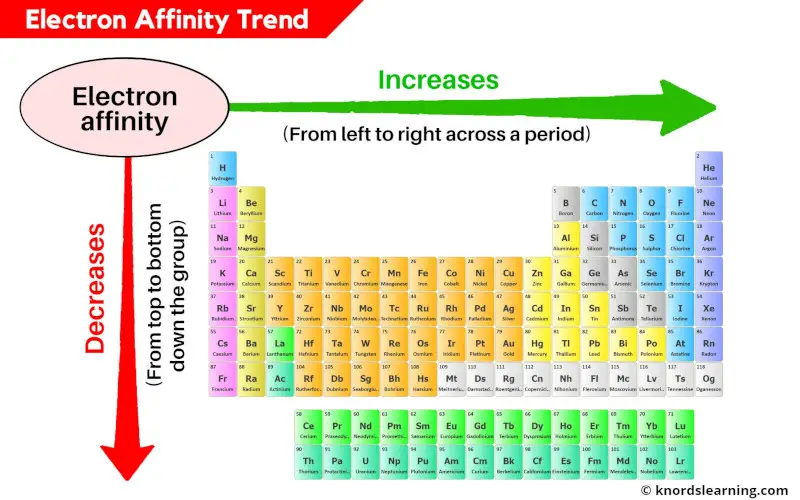
Across a Period (Left to Right):
- General Increase: Electron affinity generally increases as you move from left to right across a period. This is due to the increasing nuclear charge, which attracts the incoming electron more strongly.
- Exceptions: There are some exceptions to this general trend, particularly for groups 14 (carbon group) and 15 (nitrogen group). This is because these elements have half-filled or fully filled p-orbitals, which contribute to increased stability and a lower tendency to gain an electron.
Down a Group (Top to Bottom):
- General Decrease: Electron affinity generally decreases as you move down a group. This is because the incoming electron is added to an orbital further away from the nucleus, experiencing less attraction from the nucleus. Additionally, shielding by inner electrons weakens the nuclear attraction.
Factors Affecting Electron Affinity:
- Nuclear Charge: A higher nuclear charge leads to a stronger attraction for the incoming electron, resulting in a higher EA.
- Atomic Size: Smaller atoms have a stronger attraction for the incoming electron, leading to a higher EA.
- Electron Configuration: Atoms with half-filled or fully filled orbitals tend to have lower EAs due to their increased stability.
Understanding the Importance of Electron Affinity:
- Predicting Reactivity: Electron affinity plays a crucial role in determining an element’s reactivity. Elements with high electron affinities tend to gain electrons readily, becoming strong oxidizing agents. Conversely, elements with low electron affinities are less likely to gain electrons and act as reducing agents.
- Bond Formation: The difference in electron affinities between two atoms influences the type of chemical bond formed. For example, a large difference in electron affinity leads to ionic bonds, while a smaller difference favors covalent bonds.
- Understanding Chemical Reactions: By considering electron affinity, we can predict the likelihood of reactions occurring and the type of products formed.
Related Searches:
1. Electron Affinity Trends and Exceptions:
- Understanding the Exceptions: While the general trends in electron affinity are helpful, exceptions arise due to factors like electron configuration and shielding effects. For instance, nitrogen (N) has a lower EA than oxygen (O) because of its half-filled p-orbitals.
- Electron Configuration and Stability: The stability of an atom’s electron configuration significantly influences its electron affinity. Atoms with half-filled or fully filled orbitals tend to be more stable, leading to lower EAs.
- Shielding Effects: As you move down a group, the number of inner electrons increases, shielding the incoming electron from the nucleus, reducing the attraction and leading to a lower EA.
2. Electron Affinity and Ionization Energy:
- Relationship and Differences: Electron affinity and ionization energy (IE) are closely related. While EA measures the energy change upon adding an electron, IE measures the energy required to remove an electron. Both are crucial for understanding an element’s tendency to gain or lose electrons.
- Trends and Correlations: Both EA and IE generally increase across a period and decrease down a group. This correlation arises from the same fundamental factors: nuclear charge and atomic size.
3. Electron Affinity and Electronegativity:
- Electronegativity and Bonding: Electronegativity measures an atom’s ability to attract electrons within a bond. It is closely related to electron affinity, as both reflect an atom’s tendency to gain electrons.
- Relationship and Differences: While both electronegativity and electron affinity indicate an atom’s ability to attract electrons, electronegativity specifically considers the atom’s environment within a bond, whereas electron affinity focuses on the isolated atom.
4. Electron Affinity and Chemical Bonding:
- Ionic Bonds: A significant difference in electron affinity between two atoms leads to the formation of ionic bonds, where one atom gains an electron and becomes negatively charged, while the other loses an electron and becomes positively charged.
- Covalent Bonds: When the difference in electron affinity between two atoms is smaller, they share electrons, forming covalent bonds.
5. Electron Affinity and Reactivity:
- Oxidizing Agents: Elements with high electron affinities readily gain electrons and act as oxidizing agents, causing other species to lose electrons.
- Reducing Agents: Elements with low electron affinities are less likely to gain electrons and act as reducing agents, causing other species to gain electrons.
6. Electron Affinity and Periodic Table Trends:
- Periodic Trends Visualization: The periodic table can be used to visualize the trends in electron affinity. Elements with high EAs are located in the upper right corner, while those with low EAs are found in the lower left corner.
- Predicting Chemical Behavior: Understanding the periodic trends in electron affinity allows us to predict the chemical behavior of elements and their interactions.
7. Electron Affinity and Applications:
- Material Science: Electron affinity plays a crucial role in the development of new materials with specific properties. For example, elements with high electron affinities are used in solar cells and semiconductors.
- Chemistry Research: Electron affinity is a fundamental property studied in various fields of chemistry, including electrochemistry, spectroscopy, and theoretical chemistry.
8. Electron Affinity and Quantum Chemistry:
- Theoretical Calculations: Quantum chemistry methods are used to calculate electron affinities, providing insights into the electronic structure and bonding properties of atoms and molecules.
- Computational Chemistry: Computational chemistry models allow us to predict and understand the behavior of molecules and reactions based on their electron affinity and other properties.
FAQs about Periodic Trends in Electron Affinity:
1. What is the most electronegative element?
Fluorine (F) is the most electronegative element, with the highest electron affinity.
2. Why does nitrogen have a lower electron affinity than oxygen?
Nitrogen has a lower electron affinity than oxygen because nitrogen has a half-filled p-orbital, which is more stable than the partially filled p-orbital of oxygen.
3. How does electron affinity differ from ionization energy?
Electron affinity measures the energy change when an electron is added to an atom, while ionization energy measures the energy required to remove an electron from an atom.
4. How does electron affinity relate to chemical bonding?
The difference in electron affinity between two atoms influences the type of bond formed between them. A large difference in electron affinity leads to ionic bonds, while a smaller difference favors covalent bonds.
5. How can we predict the electron affinity of an element?
While predicting the exact electron affinity value is complex, understanding the periodic trends and factors affecting electron affinity can help us make predictions about the relative electron affinity of elements.
Tips for Understanding Periodic Trends in Electron Affinity:
- Focus on the General Trends: Remember that electron affinity generally increases across a period and decreases down a group.
- Consider Exceptions: Be aware of the exceptions to the general trends, particularly for groups 14 and 15.
- Relate Electron Affinity to Other Properties: Connect electron affinity to other periodic properties like electronegativity and ionization energy.
- Visualize the Trends: Use the periodic table to visualize the trends in electron affinity, helping you remember the patterns.
- Practice with Examples: Work through examples to solidify your understanding of electron affinity and its relationship to chemical behavior.
Conclusion
Understanding the periodic trends in electron affinity is essential for comprehending the reactivity and bonding behavior of elements. By grasping the factors influencing electron affinity and its relationship to other periodic properties, we can predict chemical reactions and understand the formation of various chemical bonds. Electron affinity remains a fundamental concept in chemistry, providing a key tool for understanding the fascinating world of atoms and molecules.




Closure
Thus, we hope this article has provided valuable insights into Understanding the Periodic Trends in Electron Affinity: A Comprehensive Guide. We thank you for taking the time to read this article. See you in our next article!
.PNG)