Understanding Periodic Trends: Electron Affinity and its Significance
Understanding Periodic Trends: Electron Affinity and its Significance
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Understanding Periodic Trends: Electron Affinity and its Significance. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding Periodic Trends: Electron Affinity and its Significance

The periodic table, a cornerstone of chemistry, organizes elements based on their recurring properties. One such property, electron affinity, plays a crucial role in chemical bonding and reactivity. Electron affinity refers to the change in energy when an atom gains an electron in its gaseous state. This seemingly simple concept reveals intricate patterns across the periodic table, providing valuable insights into the behavior of elements.
Periodic Trends in Electron Affinity
Electron affinity exhibits distinct trends across the periodic table, influenced by factors such as nuclear charge, electron shielding, and atomic size.
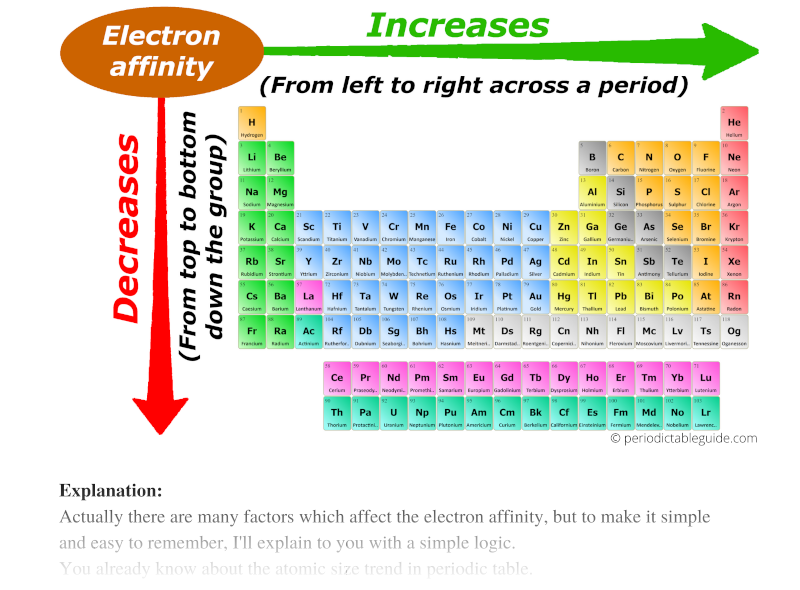
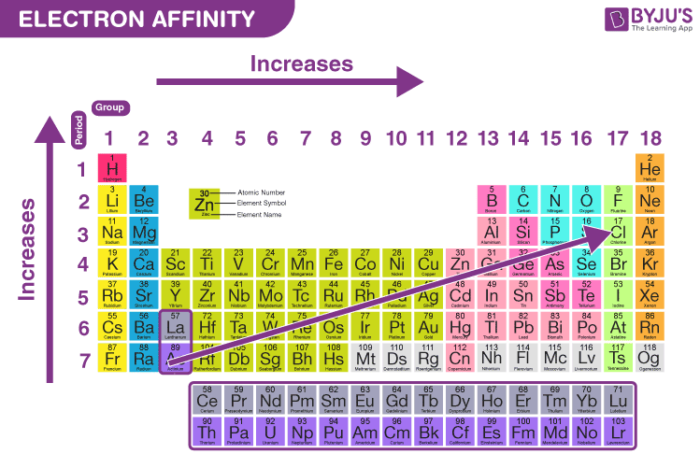
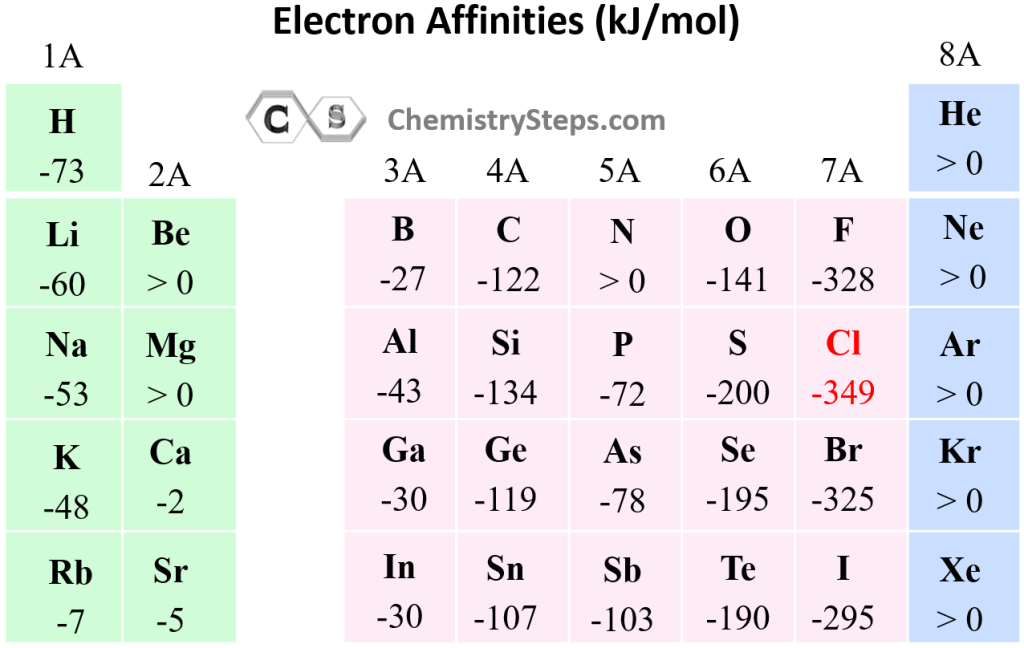
- Across a Period (Left to Right): Generally, electron affinity increases as you move from left to right across a period. This is because elements on the right side of the periodic table have a stronger attraction for electrons due to their higher nuclear charge. With more protons in the nucleus, the electrons are more tightly held, making it energetically favorable for them to gain an additional electron.
- Down a Group (Top to Bottom): Electron affinity generally decreases as you move down a group. While the nuclear charge increases, the added electron shells provide increased shielding, reducing the effective nuclear charge experienced by the outermost electron. This weaker attraction makes it less favorable for the atom to gain an electron.
Exceptions to the Trends
It’s important to acknowledge that there are exceptions to these general trends. For instance, the electron affinity of nitrogen is lower than that of oxygen, despite both being in the same period. This anomaly is attributed to the half-filled p-orbital configuration of nitrogen, which is more stable than the partially filled p-orbital configuration of oxygen.
Importance of Electron Affinity
Understanding electron affinity is essential for several reasons:
- Predicting Chemical Reactivity: Elements with high electron affinity tend to be strong oxidizing agents, readily accepting electrons to form negative ions. This property is crucial in understanding redox reactions, which form the basis of many chemical processes.
- Formation of Ionic Compounds: The difference in electron affinity between two elements plays a significant role in the formation of ionic compounds. Elements with high electron affinity readily accept electrons from elements with low electron affinity, resulting in the formation of oppositely charged ions that attract each other.
- Understanding Bonding: Electron affinity influences the type of bond that forms between atoms. Elements with high electron affinity tend to form ionic bonds, while elements with low electron affinity typically form covalent bonds.
Related Searches
- Electron Affinity and Electronegativity: While both concepts relate to an atom’s ability to attract electrons, electron affinity refers to the energy change upon gaining an electron, while electronegativity measures the relative ability of an atom to attract electrons within a bond.
- Electron Affinity and Ionization Energy: Ionization energy is the energy required to remove an electron from an atom. Electron affinity and ionization energy are inversely related. Elements with high electron affinity typically have low ionization energy, meaning they readily gain electrons but lose them less easily.
- Electron Affinity and Chemical Bonding: Electron affinity plays a crucial role in determining the type of chemical bond formed between atoms. Elements with high electron affinity tend to form ionic bonds, while elements with low electron affinity typically form covalent bonds.
- Electron Affinity and Periodic Table Trends: Understanding the trends in electron affinity across the periodic table is crucial for predicting the reactivity and bonding behavior of elements.
- Electron Affinity and Reactivity: Elements with high electron affinity are generally more reactive, as they readily accept electrons to form stable ions.
- Electron Affinity and Oxidation States: Electron affinity influences the oxidation states an element can adopt. Elements with high electron affinity tend to have negative oxidation states.
- Electron Affinity and Redox Reactions: Electron affinity plays a key role in redox reactions, which involve the transfer of electrons. Elements with high electron affinity act as oxidizing agents, accepting electrons.
- Electron Affinity and Chemical Properties: Electron affinity is a fundamental chemical property that significantly influences an element’s reactivity, bonding behavior, and overall chemical properties.
FAQs
Q: What is the significance of electron affinity in chemistry?
A: Electron affinity is a key property that influences an element’s reactivity, bonding behavior, and ability to form ionic compounds. It helps predict how elements will interact with each other and participate in chemical reactions.
Q: How does electron affinity relate to electronegativity?
A: While both concepts relate to an atom’s ability to attract electrons, electron affinity refers to the energy change upon gaining an electron, while electronegativity measures the relative ability of an atom to attract electrons within a bond.
Q: Why does electron affinity generally increase across a period?
A: As you move across a period, the nuclear charge increases, while the number of electron shells remains the same. This stronger attraction between the nucleus and electrons makes it more favorable for the atom to gain an electron, leading to a higher electron affinity.
Q: Why does electron affinity generally decrease down a group?
A: As you move down a group, the nuclear charge increases, but the added electron shells provide increased shielding. This reduces the effective nuclear charge experienced by the outermost electron, making it less favorable for the atom to gain an electron, resulting in a lower electron affinity.
Q: Are there any exceptions to the periodic trends in electron affinity?
A: Yes, there are exceptions to the general trends. For example, nitrogen has a lower electron affinity than oxygen, despite being in the same period. This is due to nitrogen’s half-filled p-orbital configuration, which is more stable than the partially filled p-orbital configuration of oxygen.
Tips
- Visualize the Periodic Table: Use a periodic table to visualize the trends in electron affinity. Focus on how the values change as you move across periods and down groups.
- Consider the Factors: Remember that electron affinity is influenced by nuclear charge, electron shielding, and atomic size. Consider these factors when analyzing the trends.
- Practice with Examples: Practice applying the concept of electron affinity to different elements and predict their reactivity based on their position in the periodic table.
Conclusion
Electron affinity is a fundamental chemical property that plays a crucial role in understanding the behavior of elements and their interactions. By understanding the trends in electron affinity across the periodic table, we gain valuable insights into the reactivity, bonding behavior, and chemical properties of elements. This knowledge is essential for predicting chemical reactions, designing new materials, and advancing our understanding of the world around us.
.PNG)



Closure
Thus, we hope this article has provided valuable insights into Understanding Periodic Trends: Electron Affinity and its Significance. We thank you for taking the time to read this article. See you in our next article!
