Understanding Basicity Trends in the Periodic Table: A Comprehensive Guide
Understanding Basicity Trends in the Periodic Table: A Comprehensive Guide
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Understanding Basicity Trends in the Periodic Table: A Comprehensive Guide. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Understanding Basicity Trends in the Periodic Table: A Comprehensive Guide
- 2 Introduction
- 3 Understanding Basicity Trends in the Periodic Table: A Comprehensive Guide
- 3.1 Basicity Trends: A Journey Through the Periodic Table
- 3.2 Applications of Basicity Trends
- 3.3 Related Searches
- 3.4 FAQs
- 3.5 Tips for Understanding Basicity Trends
- 3.6 Conclusion
- 4 Closure
Understanding Basicity Trends in the Periodic Table: A Comprehensive Guide
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
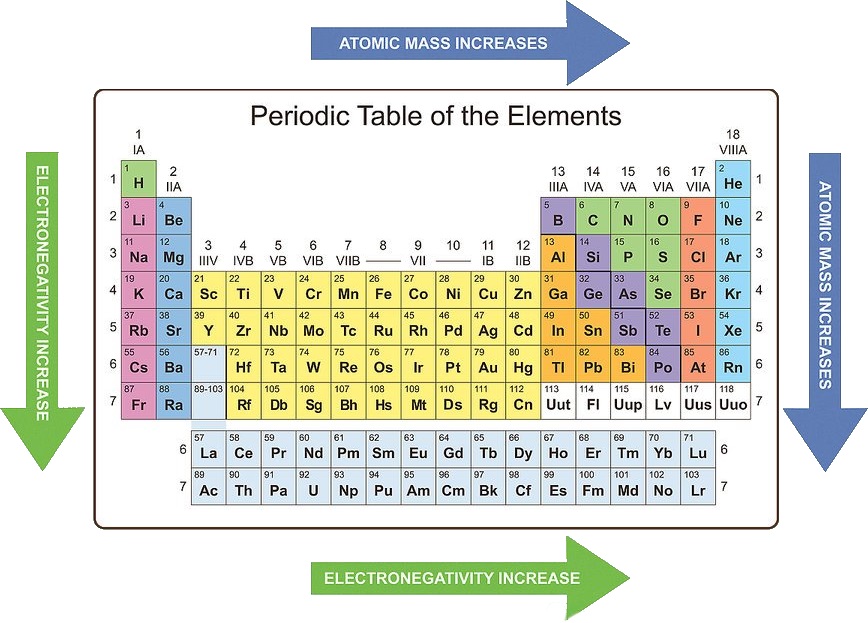
The periodic table, a cornerstone of chemistry, organizes elements based on their recurring properties. One such property, basicity, refers to the ability of a substance to accept protons (H+) and form a bond with them. Understanding basicity trends within the periodic table allows chemists to predict the behavior of elements and compounds, guiding the development of new materials and processes.
Basicity Trends: A Journey Through the Periodic Table
Basicity trends in the periodic table are influenced by several factors, including:
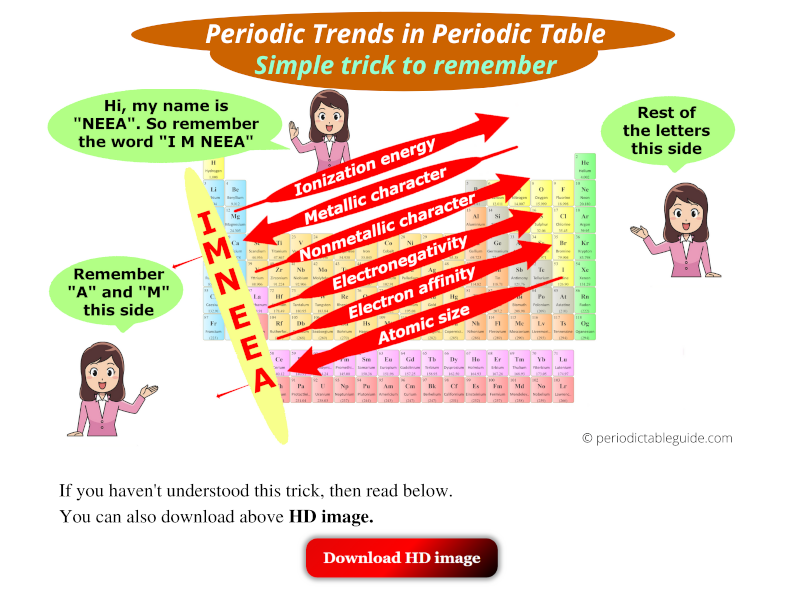
- Electronegativity: Elements with lower electronegativity tend to be more basic, as they readily donate electrons and form bonds with protons. Moving down a group in the periodic table, electronegativity decreases, leading to increased basicity.
- Size of the atom: Larger atoms have a weaker hold on their electrons, making them more likely to donate electrons and exhibit higher basicity.
- Ionic character: Compounds with more ionic character tend to be more basic, as the negatively charged ion readily accepts protons.
Key Trends in Basicity:
- Across a Period: Basicity generally decreases across a period from left to right. This is due to increasing electronegativity and a smaller atomic size.
- Down a Group: Basicity generally increases down a group. This is due to decreasing electronegativity and a larger atomic size.
Examples of Basicity Trends:
- Group 1 and 2 Elements: The alkali metals (Group 1) and alkaline earth metals (Group 2) are highly basic due to their low electronegativity and large atomic size.
- Group 15 and 16 Elements: Elements like nitrogen (N) and oxygen (O) are less basic than phosphorus (P) and sulfur (S) due to their higher electronegativity and smaller atomic size.
- Transition Metals: Transition metals exhibit a wide range of basicity depending on their oxidation state and the nature of the ligand.
Applications of Basicity Trends
Understanding basicity trends has numerous applications in various fields:
- Chemistry: Basicity is crucial in predicting the reactivity of compounds and designing new catalysts.
- Material Science: Basicity plays a role in the synthesis and properties of materials like ceramics, polymers, and composites.
- Biology: Basicity is essential for understanding the behavior of biological molecules like amino acids and proteins.
- Environmental Science: Basicity is relevant in analyzing the pH of water bodies and soil, impacting environmental health.
Related Searches
1. Basicity of Oxides: The basicity of oxides generally increases down a group and decreases across a period. For example, Na2O is more basic than MgO, while MgO is more basic than Al2O3.
2. Basicity of Hydroxides: Hydroxides of metals are generally basic, while hydroxides of nonmetals are acidic. For instance, NaOH is a strong base, while HCl is a strong acid.
3. Basicity of Amines: Amines, containing the -NH2 group, exhibit basicity due to the lone pair of electrons on the nitrogen atom. The basicity of amines increases with the electron-donating ability of substituents.
4. Basicity of Amides: Amides, containing the -CONH2 group, are less basic than amines due to the electron-withdrawing effect of the carbonyl group.
5. Lewis Basicity: Lewis bases are electron-pair donors. The ability of a Lewis base to donate electrons is referred to as its Lewis basicity.
6. Brønsted-Lowry Basicity: Brønsted-Lowry bases are proton acceptors. The ability of a Brønsted-Lowry base to accept protons is referred to as its Brønsted-Lowry basicity.
7. Basicity of Anions: Anions are generally basic, as they have a negative charge and can readily accept protons.
8. Basicity of Cations: Cations are generally acidic, as they have a positive charge and can donate protons.
FAQs
Q1. What are the factors that affect basicity?
A: The factors influencing basicity include electronegativity, atomic size, ionic character, and the presence of electron-donating or electron-withdrawing groups.
Q2. How does basicity relate to pH?
A: Basic solutions have a higher pH than acidic solutions. The pH scale measures the concentration of hydrogen ions (H+), with higher pH indicating lower H+ concentration and therefore higher basicity.
Q3. How can basicity be measured?
A: Basicity can be measured through various methods, including:
- Titration: A solution of known concentration is added to a solution of unknown concentration until the reaction is complete, allowing for the calculation of the basicity.
- pH meter: A pH meter directly measures the pH of a solution, indicating its basicity.
- Spectroscopic techniques: Techniques like infrared spectroscopy and nuclear magnetic resonance spectroscopy can provide insights into the basicity of molecules.
Q4. What is the importance of basicity in chemistry?
A: Basicity is crucial for understanding the reactivity of compounds, designing new catalysts, and synthesizing materials with specific properties.
Q5. How can basicity be used to predict the behavior of elements and compounds?
A: By understanding the trends in basicity across the periodic table, chemists can predict how different elements and compounds will react with each other. This knowledge is essential for developing new materials and processes.
Tips for Understanding Basicity Trends
- Use the periodic table as a visual aid: The periodic table clearly shows the trends in electronegativity and atomic size, which directly impact basicity.
- Focus on the key groups and periods: Pay attention to the trends in basicity within Group 1, 2, 15, and 16 elements, as well as across periods.
- Consider the influence of substituents: The presence of electron-donating or electron-withdrawing groups can significantly affect the basicity of a molecule.
- Practice with examples: Applying the concepts to real-world examples helps solidify your understanding of basicity trends.
Conclusion
Basicity trends are an essential aspect of understanding the behavior of elements and compounds. By recognizing the factors influencing basicity and the patterns observed across the periodic table, chemists can predict reactivity, design new materials, and advance our understanding of the chemical world. As we continue to explore the intricacies of chemistry, the knowledge of basicity trends will remain a vital tool for innovation and discovery.
/periodictrendstable-5c4a46614cedfd000187c5db.jpg)



.PNG)

Closure
Thus, we hope this article has provided valuable insights into Understanding Basicity Trends in the Periodic Table: A Comprehensive Guide. We appreciate your attention to our article. See you in our next article!